Laparoscopic cytoreductive surgery for metastatic colon cancer - how to improve treatment strategy
Andrey Atroshchenko*, Igor Khatkov, Stepan Pozdnyakov, Mikhail Danilov
Moscow Clinical Scientific Center, Moscow, Russia

Background
Colon cancer (CC) one of the most common oncological disease in the World. More than 30000 new cases were registered and 12 000 have die annually in Russia, up to 30% patients have metastatic CC at first visiting to oncologist [1].
In Russia treatment of metastatic CC is in focus of interests because there is a huge part of patients with an advanced disease. New treatment strategy and medical technology allowed expanding indications for surgery in patients with metastatic CC even with sever comorbidities. Recent studies showed the improvement of treatment results after cytoreductive surgery even in patients with multiple liver and lung metastases [2;3;4;5].
Nowadays, introduction of innovation minimally invasive surgery, advantages in molecular technology and new scheme of chemotherapy allowed extending the indication for cytoreductive surgery for metastatic CC because it could help to optimize treatment strategy and achieve the improvement of quality of life and long-term results. The laparoscopic precision technique in cytoreductive surgery could achieve the minimal level of morbidity and mortality, shorter period of rehabilitation and time to adjuvant chemotherapy after surgery [6;7].
Recently, the results of multiple multicenter randomized controlled trials (COST, Barcelona trial, CLASSIC, COLOR, COLOR-2), confirming that there is no differences in short-term and long-term results between the laparoscopic and open access surgery for patients with CC [8;9;10;11;12;13;14;15;16;17].
In modern literature there is only single articles reflecting laparoscopic cytoreductive surgery for metastatic CC.
Materials and methods
We analysis 89 patients treatment results with CC (T1–4a,Nany, M1a-b). Inclusion criteria in study: colon carcinoma with curable synchronous distant metastases (except the patients with total peritoneal carcinomatosis); metastases no more than 2 organs, ECOG <2. Exclusion criteria: ECOG >2, brain and bone metastases, retroperitoneal lymph nodes invasion, metastases more than 2 organs, tumor or metastases destruction, acute bowel obstruction, multiple synchronous or metachronous metastases. All patients underwent simultaneous or staged cytoreductive surgery and adjuvant chemotherapy. The patients were divided into 2 groups: study group (44 patients) - underwent laparoscopic cytoreductive surgery; main group - cytoreductive surgery by open access (45 patients). The groups were similar by sex, age, tumor spread and localization and histological structure, comorbidities.
Tumor localization and spread of metastases one of the most important factor determining for surgery plane (figure 1).
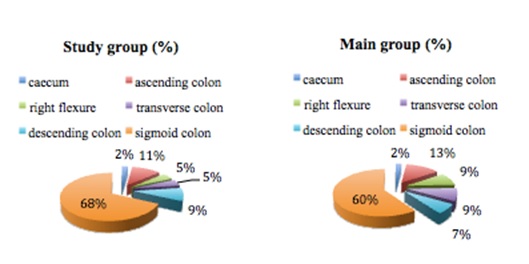
Figure 1
All patients had histologically verified colon adenocarcinoma: high grade differentiated adenocarcinoma - 10,1% patients, moderate – 76,4%, low-grade – 6,7% and 6,7% patients had mucous adenocarcinoma. Main part of the patients in our study had moderate–differentiated carcinoma with T3 invasion.
The lymph node metastases were detected in 75% patients study group, and 82.2%– in main group.
The distribution of affected organs metastases and localization is presented in table 1.
Table 1
The number and metastases localization
|
Metastases localization |
Study group |
Main group |
|
N (%) |
N (%) |
|
|
Liver all |
38 (86,4) |
35 (77,8) |
|
Solitary liver metastases |
6 (14,6) |
5 (13,2) |
|
Single liver metastases |
11 (26,8) |
11 (28,9) |
|
Multiple liver metastases |
24 (58,5) |
22 (57,9) |
|
Lung |
2 (4,6) |
3 (6,7) |
|
Solitary lung metastases |
0 |
1 (20,0) |
|
Single metastases of the lung |
2 (33,3) |
2 (40,0) |
|
Multiple lung metastases |
4 (66,7) |
2 (40,0) |
|
Left Lung m metastases |
1 (20,0) |
1 (16,7) |
|
Right Lung metastases |
2 (40,0) |
1 (16,7) |
|
Bilateral lung metastases |
2 (40,0) |
4 (66,7) |
|
Liver and Lung |
3 (6,8) |
3 (6,7) |
|
Metastases in other organs |
1 (2,3) |
4 (8,8) |
|
М1а - metastases |
39 (88,6) |
38 (84,4) |
|
М1b - metastases |
5 (11,4) |
7 (15,6) |
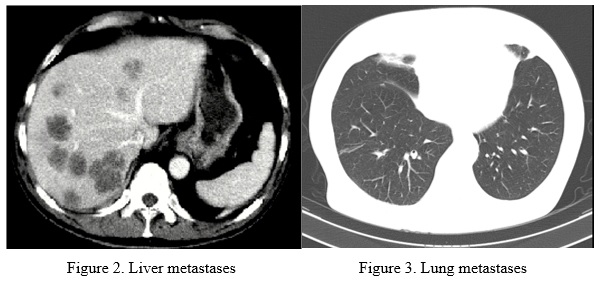
The main part of patients in both group had multiple bilateral liver metastases and multiple bilateral lung metastases (figure 2, 3).
Different symptoms of the primary tumor in the colon were observed more than in 50% of patients (table 2).
Table 2
Primary tumor complications
|
Type of complications |
Study group, N (%) |
Main group, |
|
Bowel obstruction subclinical |
14 (31,8) |
15 (33,3) |
|
Blood in stool |
7 (15,9) |
7 (15,6) |
|
Toxicoanemic syndrome |
3 (6,8) |
5 (11,1) |
|
Total |
24 (54,6) |
27 (60,0) |
Almost 91% patients in study group and 82% patients in main group had severe comorbidities (table 3).
Table 3
Comorbidities
|
Comorbidities |
Study group |
Main group N |
|
Coronary heart disease |
28 |
25 |
|
Arterial hypertension of the I st |
31 |
29 |
|
Arrhythmia |
2 |
1 |
|
Diabetes |
5 |
4 |
|
Obesity (BMI 25-39,9) |
30 |
34 |
|
Chronic obstructive pulmonary disease |
2 |
3 |
|
Gastrointestinal tract benign disease |
12 |
8 |
Results
All patients (89) underwent cytoreductive surgery with primary tumor or primary tumor and distant metastases resection (table 4).
Table 4
Characteristic of colon surgery procedure
|
Type of surgery |
Study group |
Main group, |
|
Right hemicolectomy |
9 (20,5) |
13 (28,9) |
|
Left hemicolectomy |
4 (9,1) |
4 (8,9) |
|
Sigmoid resection |
24 (54,6) |
18 (40,0) |
|
Rectal resection |
3 (6,8) |
2 (4,4) |
|
Hartman procedure |
4 (9,1) |
8 (17,8) |
|
Total: |
44 (100) |
45 (100) |
Hartmann's procedure underwent 4 patients in study group and 8 patients in main group because had subclinical sights of bowel obstruction and colon wall hypertrophy. Colon anastomosis formed in 90,9% cases in study group and 82.2%- in main group.
Simultaneous R0 resection performed in cases when the general condition (ECOG 0-2; APACHE II - 0-14 score) of patient, number and localization of metastases allowed to do it with low risk of morbidity and mortality (table 5).
Table 5
Characteristics of surgery procedure for metastases
|
Surgery for metastases |
Study group |
Main group, |
|
Simultaneus R-0 resection |
||
|
Liver resection |
1 (2,3) |
0 (0) |
|
Salpingo-oophorectomy |
1 (2,3) |
0 (0) |
|
Hysterectomy |
0 (0) |
1 (2,2) |
|
Omentum resection |
0 (0) |
1 (2,2) |
|
Staged R-0 resection |
||
|
Lung resection |
1 (2,3) |
2 (4,4) |
|
Liver resection: |
10 (22,7) |
7 (15,6) |
|
Right hemihepatectomy |
4 (9,1) |
2 (4,4) |
|
Left hemihepatectomy |
2 (4,5) |
1 (2,2) |
|
Atypical liver resection |
4 (9,1%) |
4 (8,9%) |
|
Total R-0 resection: |
13 (29,6%) |
11 (24,4%) |
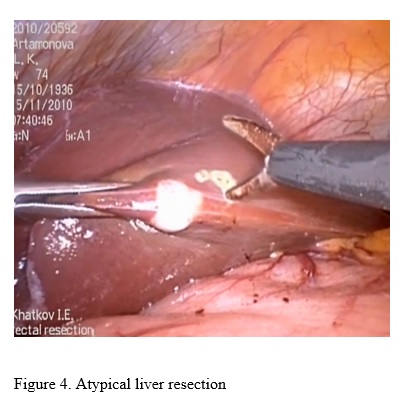
R-0 resection performed 29,6% of patients in study group and 24,4% - in main group (figure 4).
The average number of lymph node removal was similar in both groups (13 and 12 respectively, p<0.05 (figure 5).
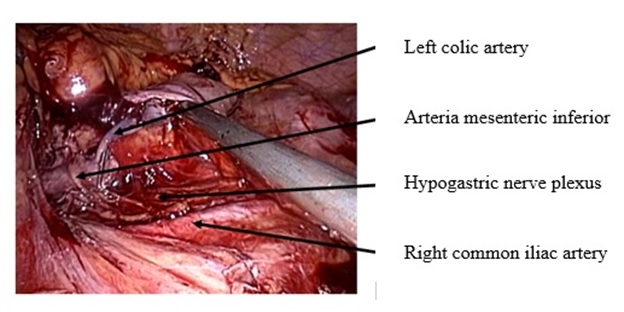
Figure 5. D-3 lymph node dissection with left colic artery preservation
The average time duration of the operation in study group was 210 min, in main group - 120 min. Surgery time depends on surgeons learning curve, and have tended to decline after 30 laparoscopic procedures.
In obese patients the time of surgery significantly longer especially in laparoscopic access group because it so difficult to do the navigation and visualization of anatomical structures: obesity I degree – 234,4 min; II degree of obesity – 295 min. In control group: I degree obesity – 163,6 min; obesity II degree – 226,7 min..
We analyzed intraoperative blood loss as a factor of surgical trauma. The average blood loss in study group was 300 ml, in control group – 1200 ml p<0.05. The data shows that, the laparoscopic procedure could help to reduced operative blood loss significantly and, consequently, reduced the number of transfusion complications and speed up patients rehabilitation.
Surgical complications were similar in both group: 5% in laparoscopic access group and 4% - in open surgery group p>0.60. The main surgical complication was hemorrhage.
We studded several indicators during early postoperative period: time to peristalsis recovery first stool, first food taken, time to patient activation, painkillers intake, postoperative complications, hospital stay, and time to start adjuvant chemotherapy. All of this parameters had improved in study group: time to peristalsis restoration was shorter in 2,5 times (p<0.05), and admitted reduction time of first stool in 1.4 times (p=0,05), (table 6).
Table 6
Time to bowel movement restoration
|
Settings |
Study group, (days) |
Main group |
Р |
|
First peristalsis |
1,8 ± 0,7 |
4,51 ± 1,5 |
<0,05 |
|
First stool |
3,4 ± 1,3 |
4,78 ± 1,4 |
=0,05 |
The quality of life in early postoperative period is an important parameter, which determined by the first food taken, bowel movement restoration, and time to painkillers intake. The results also confirm the improvement quality of life in early postoperative period in patients undergoing laparoscopic procedures (table 7).
Table 7
Quality of life in early postoperative period
|
Settings |
Study group, days (median) |
Main group, days, (median) |
Р |
|
Average time to patient activation |
2,2 ± 1,1 (2) |
3,9 ± 0,9(4) |
<0,05 |
|
Painkillers intake |
2,3 ± 1,4 (2) |
4,4 ± 1,4 (4) |
0,05 |
|
First food taken |
2,9 ± 0,7 (3) |
3,9 ± 1,3 (4) |
<0,05 |
Precision laparoscopic technique allows significantly speed up the postoperative patient rehabilitation in comparison with open surgery.
Postoperative complications in main group have noted in 8 (17.8 %) patients, 3 patients of them was re-operated. In study group complications were noted in 3 patients (6.8%), 2 of them - re-operated. The results showed that the laparoscopic access could minimize postoperative complications.
Hospital stay after laparoscopic surgery were shorter: 9 days in study group versus 13 days in main group (p<0.05). The laparoscopic precision technique could speed up patients rehabilitation up to 1.5 times.
Adjuvant chemotherapy plays a key role in treatment strategy for patients with metastatic CC. Time to start chemotherapy after surgery – one of the most important indicator, which often depends on postoperative rehabilitation. The surgical procedures and postoperative complications - main factors that prolonged time to start adjuvant chemotherapy. Well known that the early start of chemotherapy after cytoreductive surgery is beneficial to the long-terms outcomes. Short period of rehabilitation after laparoscopic surgery, improving the quality of life, lower percentages of postoperative complications allowed to reduce time to start the adjuvant chemotherapy: 14.7 days – study group; 27,5– main group (p<0.05).
The results of 2-year overall survival after cytoreductive surgery by laparoscopic access and open surgery are comparable (69,5% and 61,6%, respectively, p=0,96 (figure 6).
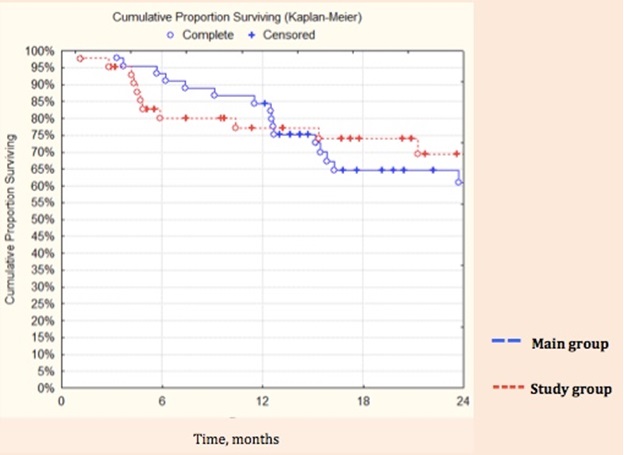
Figure 5. Kaplan-Meyer 2-year survival
During 2 years follow up we did not registered any cases of local recurrence.
Conclusion
The results of our study showed that the laparoscopic cytoreductive surgery for patients with metastatic CC is safe, could minimize surgical trauma and speed up postoperative recovery even in patients with multiple metastases and severe comorbidity. The laparoscopic access indicated for patients with metastatic colon cancer stage T1-4a Ndifferent M1a-b. Contraindications for laparoscopic surgery are locally advanced huge tumors which invades into the adjacent organs aor structures (T4b) and advanced peritoneal carcinomatosis or adhesions. The introduction of minimally invasive laparoscopic technology allows extending the indications for cytoreductive surgery even in patients with metastatic CC and severe comorbidities.
Literature
1. Aleksandrov V. B. Laparoscopic technology in colorectal surgery. – M., 2003.
2. Davydov M. I., Aksel E. M. Statistics of malignant neoplasms in Russia and CIS countries in 2007. – M., 2009.
3. Lutsevich O. E., Vtorenko I. V., Gallyamov E. A., etc. Laparoscopy in emergency surgery: current state of the problem. // The health of the capital. VII Moscow Assembly.: SB. Scientific. Works. – M., 2008. - M.: GEOS, 2008.
4. Khatkov I. E., tsvirkun V. V., Agapov V. K., Izrailov R. E. Laparoscopic pancreatoduodenectomy.// The annals of surgical Hepatology. – M., 2008. So 13, the - 3. - P. 78.
5. Bajetta E., Floriani I., Bartolomeo M. D., et al. Intergroup Trial of Adjuvant Chemotherapy in Adenocarcinoma of the Stomach (ITACA-S) trial: Comparison of a sequential treatment with irinotecan (CPT-11) plus 5-fluorouracil (5-FU)/folinic acid (LV) followed by docetaxel and cisplatin versus a 5-FU/LV regimen as postoperative treatment for radically resected gastric cancer. J Clin Oncol 30, 2012 (suppl; abstr LBA4001).
6. Bang Y. J., Kim Y. W., Yang H. K., et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomized controlled trial. Lancet 2012; 379: 315-321.
7. Gouvas N, Agalianos C, Papaparaskeva K. Surgery along the embryological planes for colon cancer: a systematic review of complete mesocolic excision // Int J Colorectal Dis. 2016 Sep;31(9):1577-94
8. Hohenberger W., Weber K., Matzel K. et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation– technical notes and outcome. Colorectal Dis. – 2009 May;11 (4):354-64; discussion 364-5.
9. Leung K.L., Lee J.F., Yiu R.Y., et al. Simultaneus laparoscopic resection of rectal cancer and liver metastasis. // J. Laparoendosc. Adv. Surg. Tech. A. 2006. Oct.; 16(5):486-8.
10. Liang J. Primacy of surgery for colorectal cancer outcomes // BJS. – 2015. – № 1. – Р. 45-48.Marks J.H., Kawun U.B., Hamdan W. et al. Redefining contraindications to laparoscopic colorectal resection for high-risk patients. // Surg. Endosc. 2008 Aug.; 22(8): 1899-904.
11. Mori S, Kita Y, Baba K. Laparoscopic complete mesocolic excision via combined medial and cranial approaches for transverse colon cancer // Surg Today. – 2016. – Aug 26.
12. Milsom J.W., Kim S.H. Laparoscopic versus open surgery for colorectal cancer. // World J. Surg. 1997; 27: 702-705.
13. Miyamoto Y. Laparoscopic resection of primary tumor for stage IV colorectal cancer. // 19-th International Congress of the EAES, A:0133, 2011.
14. Ng D.C., Co C.S., Cheung H.Y., Chung C.C., Li M.K. The outcome of laparoscopic colorectal resection in T4 cancer. // Colorectal Dis. 2011. Oct.; 13(10): e349-52. (2011).
15. Soop M., Nelson H. Is laparoscopic resection appropriate for colorectal adenocarcinoma? // Adv. Surg. 2008; 42: 205-17.
16. Kuhry E., Schwenk W., Gaupset R. et al. Long-term results of laparoscopic colorectal cancer resection. // Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No.: CD003432. DOI: 10.1002/14651858.CD003432.pub2.